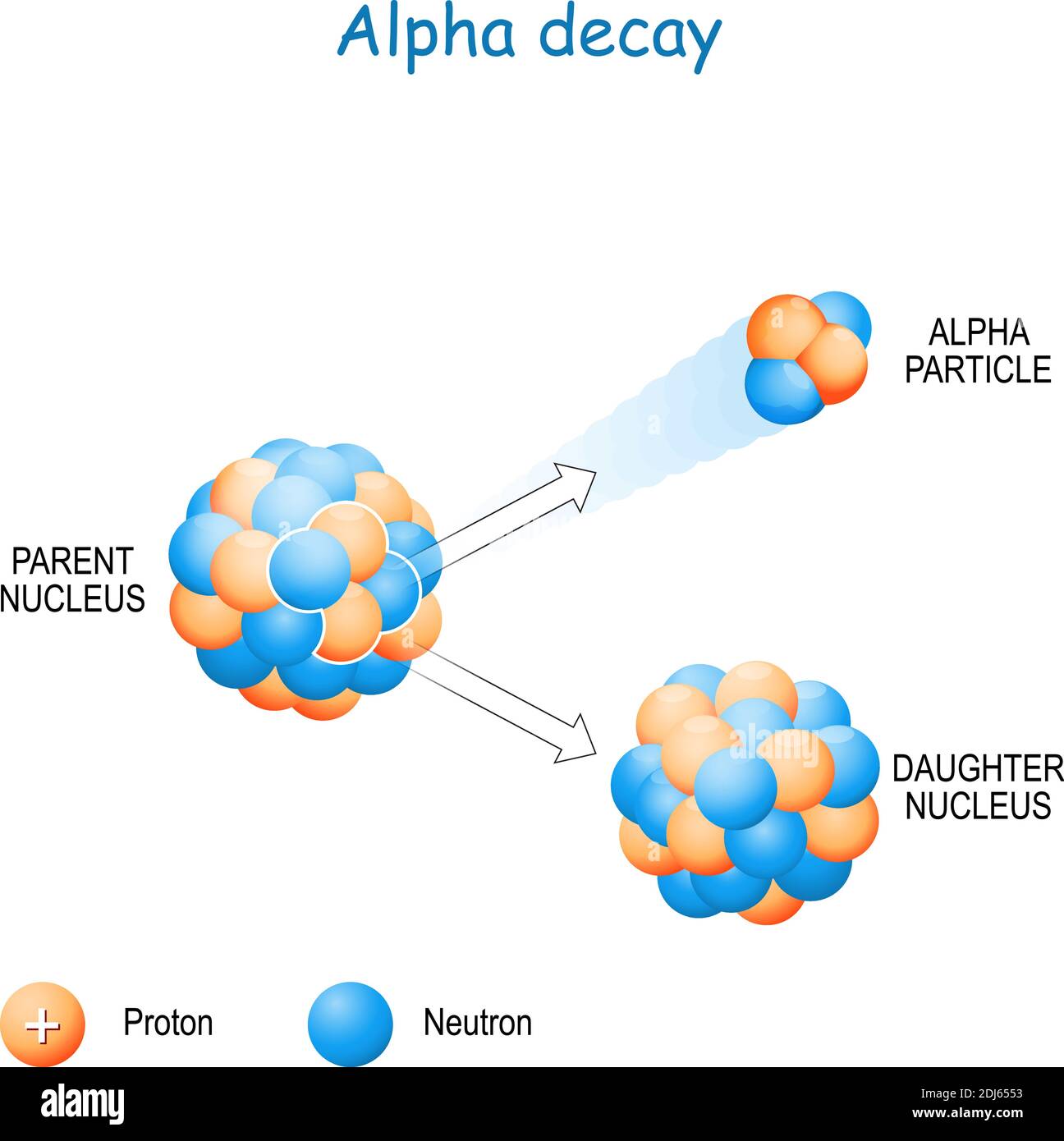
What Is The Importance Of Radioactive Decay . A process by which an element is converted into a lighter element through the. This nuclear process plays a crucial. This emission of energy is by a process known as radioactive decay. Radioactive decay, also known as nuclear decay, radioactivity, or radioactive disintegration, is a fundamental process in. There are only certain combinations of neutrons and protons, which form stable nuclei. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. Radioactive decay occurs for all nuclei with \(z > 82\),. As the number of protons increases, an increasing ratio of neutrons to protons is needed. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay.
from www.alamy.com
Radioactive decay occurs for all nuclei with \(z > 82\),. There are only certain combinations of neutrons and protons, which form stable nuclei. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. This emission of energy is by a process known as radioactive decay. A process by which an element is converted into a lighter element through the. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. As the number of protons increases, an increasing ratio of neutrons to protons is needed. When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. This nuclear process plays a crucial. Radioactive decay, also known as nuclear decay, radioactivity, or radioactive disintegration, is a fundamental process in.
Radioactive decay diagram hires stock photography and images Alamy
What Is The Importance Of Radioactive Decay Radioactive decay occurs for all nuclei with \(z > 82\),. Radioactive decay occurs for all nuclei with \(z > 82\),. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. Radioactive decay, also known as nuclear decay, radioactivity, or radioactive disintegration, is a fundamental process in. When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. There are only certain combinations of neutrons and protons, which form stable nuclei. A process by which an element is converted into a lighter element through the. As the number of protons increases, an increasing ratio of neutrons to protons is needed. This nuclear process plays a crucial. This emission of energy is by a process known as radioactive decay. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones.
From www.youtube.com
why radioactive decay law has great importance in physics3 great What Is The Importance Of Radioactive Decay The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. There are only certain combinations of neutrons and protons, which form stable nuclei. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. This emission of energy is by a. What Is The Importance Of Radioactive Decay.
From www.coursehero.com
HalfLife and Activity Physics Course Hero What Is The Importance Of Radioactive Decay This emission of energy is by a process known as radioactive decay. A process by which an element is converted into a lighter element through the. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable. What Is The Importance Of Radioactive Decay.
From owlcation.com
What Is Nuclear Radiation? Owlcation What Is The Importance Of Radioactive Decay Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. This nuclear process plays a crucial. Radioactive decay, also known as nuclear decay, radioactivity, or radioactive disintegration, is a fundamental process in. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all. What Is The Importance Of Radioactive Decay.
From www.nuclear-power.com
Radioactive Decay Definition, Types & Laws What Is The Importance Of Radioactive Decay Radioactive decay, also known as nuclear decay, radioactivity, or radioactive disintegration, is a fundamental process in. This nuclear process plays a crucial. Radioactive decay occurs for all nuclei with \(z > 82\),. As the number of protons increases, an increasing ratio of neutrons to protons is needed. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of. What Is The Importance Of Radioactive Decay.
From www.youtube.com
radioactive decay (applications of first order separable differential What Is The Importance Of Radioactive Decay This emission of energy is by a process known as radioactive decay. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. As the number of protons increases, an increasing ratio of neutrons to protons is needed. Radioactive decay occurs for all nuclei with \(z > 82\),. A process by which. What Is The Importance Of Radioactive Decay.
From www.slideserve.com
PPT Radioactive Decay PowerPoint Presentation, free download ID2129890 What Is The Importance Of Radioactive Decay This emission of energy is by a process known as radioactive decay. As the number of protons increases, an increasing ratio of neutrons to protons is needed. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. Radioactive decay occurs for all nuclei with \(z > 82\),. Radioactive decay,. What Is The Importance Of Radioactive Decay.
From www.slideserve.com
PPT Radioactive Decay PowerPoint Presentation, free download ID215275 What Is The Importance Of Radioactive Decay This emission of energy is by a process known as radioactive decay. Radioactive decay, also known as nuclear decay, radioactivity, or radioactive disintegration, is a fundamental process in. When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. This nuclear process plays a crucial. Radioactive decay is a physical phenomenon that involves. What Is The Importance Of Radioactive Decay.
From issuu.com
Type of Radioactive Decay by Aisyah Jahidin Issuu What Is The Importance Of Radioactive Decay Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. As the number of protons increases, an increasing ratio of neutrons to protons is needed. A process by which an element is converted into a lighter element through the. This emission of energy is by a process known as radioactive decay.. What Is The Importance Of Radioactive Decay.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps What Is The Importance Of Radioactive Decay There are only certain combinations of neutrons and protons, which form stable nuclei. The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. A process by which an element is. What Is The Importance Of Radioactive Decay.
From www.pinterest.ph
Radioactivity and the Types of Radioactive Decay Radioactive What Is The Importance Of Radioactive Decay This nuclear process plays a crucial. When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. A process by which an element is converted into a lighter element through the. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. There are only. What Is The Importance Of Radioactive Decay.
From www.youtube.com
Types of Radioactive Decay YouTube What Is The Importance Of Radioactive Decay Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. This emission of energy is by a process known as radioactive decay. Radioactive decay occurs for all nuclei with \(z > 82\),. There are only certain combinations of neutrons and protons, which form stable nuclei. When an individual nucleus transforms into. What Is The Importance Of Radioactive Decay.
From www.slideserve.com
PPT Radioactivity PowerPoint Presentation, free download ID487208 What Is The Importance Of Radioactive Decay This emission of energy is by a process known as radioactive decay. There are only certain combinations of neutrons and protons, which form stable nuclei. A process by which an element is converted into a lighter element through the. As the number of protons increases, an increasing ratio of neutrons to protons is needed. This nuclear process plays a crucial.. What Is The Importance Of Radioactive Decay.
From studiousguy.com
7 Radioactive Decay Examples in Real Life StudiousGuy What Is The Importance Of Radioactive Decay The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. This emission of energy is by a process known as radioactive decay. This nuclear process plays a crucial. Radioactive decay,. What Is The Importance Of Radioactive Decay.
From www.alamy.com
Radioactive decay diagram hires stock photography and images Alamy What Is The Importance Of Radioactive Decay Radioactive decay occurs for all nuclei with \(z > 82\),. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. This nuclear process plays a crucial. There are only certain combinations of neutrons and protons, which form stable nuclei. The naturally occurring radioactive isotopes of the heaviest elements fall into chains. What Is The Importance Of Radioactive Decay.
From rorynewscharles.blogspot.com
Describe Three Uses of Radioactive Elements What Is The Importance Of Radioactive Decay This emission of energy is by a process known as radioactive decay. Radioactive decay is a physical phenomenon that involves the spontaneous transformation of unstable atomic nuclei into more stable ones. Radioactive decay, also known as nuclear decay, radioactivity, or radioactive disintegration, is a fundamental process in. Radioactive decay occurs for all nuclei with \(z > 82\),. There are only. What Is The Importance Of Radioactive Decay.
From www.youtube.com
RADIO ACTIVITY LAW OF RADIOACTIVE DECAY DECAY IS EXPONENTIAL IN What Is The Importance Of Radioactive Decay Radioactive decay occurs for all nuclei with \(z > 82\),. This nuclear process plays a crucial. As the number of protons increases, an increasing ratio of neutrons to protons is needed. This emission of energy is by a process known as radioactive decay. A process by which an element is converted into a lighter element through the. Radioactive decay is. What Is The Importance Of Radioactive Decay.
From www.markedbyteachers.com
The Importance Of Radioactive Decay And HalfLife. GCSE Science What Is The Importance Of Radioactive Decay When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. As the number of protons increases, an increasing ratio of neutrons to protons is needed. This nuclear process plays a crucial. A process by which an element is converted into a lighter element through the. This emission of energy is by a. What Is The Importance Of Radioactive Decay.
From wisc.pb.unizin.org
Day 20 Rate of Radioactive Decay Chemistry 109, Fall 2020 What Is The Importance Of Radioactive Decay As the number of protons increases, an increasing ratio of neutrons to protons is needed. There are only certain combinations of neutrons and protons, which form stable nuclei. Radioactive decay occurs for all nuclei with \(z > 82\),. When an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. This nuclear process plays. What Is The Importance Of Radioactive Decay.